Abstract
Older patients (pts) with newly diagnosed ALL are ineligible for unmodified pediatric-based therapies. Even with age-adapted, dose-reduced regimens early death rates (ED) are considerable and overall survival (OS) poor. Thus, 412 pts treated according to the GMALL Elderly Protocol showed a CR rate of 75%, with 16% early death (ED) and the 3 years (yrs) OS was only 30%. Therefore, the GMALL study group developed a trial to evaluate Blinatumomab (Blina), a CD19 directed bi-specific T-cell engager, in sequence with chemotherapy in this patient population with high medical need (NCT 03480438). Blina replaces several cycles of chemotherapy being part of the standard GMALL protocol for older pts.
Pts aged 56 - 76 yrs with CD19-positive, Ph-neg B-precursor ALL are eligible. The primary endpoint is complete hematologic remission (CR) after induction i.e. one dose-reduced cycle chemotherapy induction (IP1) and one cycle Blina (Blina 1), and the key secondary endpoint is molecular response (table 1). The efficacy population includes pts treated with IP1 and Blina 1 or ED. The trial is ongoing and scheduled to recruit 50 pts.
After a 5-d prephase with dexamethasone (dexa) and cyclophosphamide (cyclo) pts receive dexa (10 mg/m 2 d 7-8 and 14-17), vincristine (2 mg d 7 and 14) and idarubicin (10 mg d 7 and 15) together with i.th. triple prophylaxis (d 6 and d 20) and G-CSF as IP1. Rituximab is given to pts with CD20+ ALL. Thereafter, pts with CR, CRu or PR receive Blina 1 (28 µg/d, 28 d). Pts with failure to IP1 are treated with IP2 (cytarabine, cyclo) followed by Blina 1. A dose step after one week (9µg/d to 28µg/d) is scheduled in all pts starting Blina while not in CR/CRu. Consolidation treatment consists of alternating cycles of intermediate-dose methotrexate/ PEG-asparaginase, intermediate-dose cytarabine and reinduction and 3 further cycles of Blina. This is followed by a standard maintenance (6-MP/MTX) up to a total treatment duration of 2 yrs. Pts receive up to 9 doses of i.th. triple combination. MRD is measured in bone marrow in the central reference laboratory in Kiel with quantitative IG/TR PCR according to EuroMRD standards after IP1, Blina 1, consolidation 1 and 2 and thereafter approximately every 2-3 months.
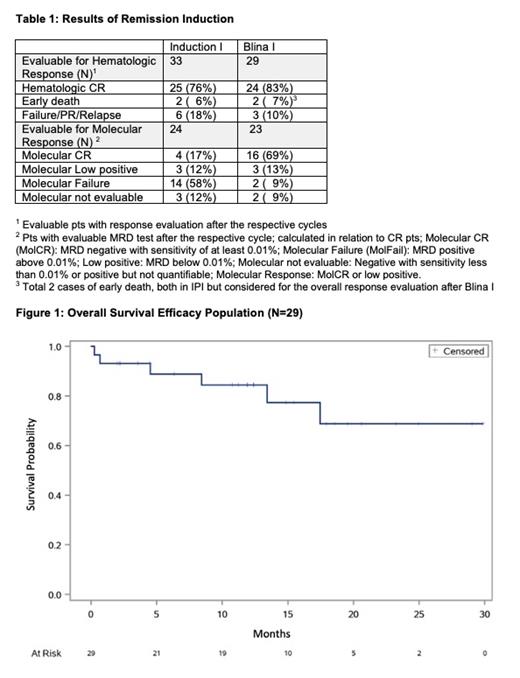
34 pts with a median age of 65 (56-76) yrs were included from 13 centers in Germany. 7 pts were older than 70 yrs (21%). 7 pts (26%) had pro-B-ALL (N=2 KMT2A rearranged) and 25 pts c-/pre-B-ALL. 47% of the pts had comorbidities according to the Charlson Score; most frequent were diabetes (18%) myocardial infarction (12%), chronic pulmonary disorder (12%). In one pt study treatment was terminated in CR due to subdural hemorrhage in IP1 considered as contraindication for Blina 1. 33 pts were evaluable for IP1: 76% achieved CR/CRu, 9% PR (N=3) 3 pts had treatment failure (9%) and 2 pts (6%) died due to infections. 29% (N=9) had a molecular response (17% MolCR) (table 1). 1/3 pts with failure after IP1 had a CR after IP2. 29 pts were evaluable for the primary endpoint after Blina 1. 24 were in hematologic CR (83%), 3 had failure (10%) and 2 experienced ED (7%; both during IP1 as mentioned above; no additional ED). 82% of the CR pts (N=19) had a molecular response (69% MolCR). 8 / 9 pts with pro-B-ALL had a CR after Blina 1 (89%) and the molecular response rate was 62% (37% MolCR).
The median follow-up is 363 (26-1001) days. Survival probability for the efficacy population (N=29) after 1 yr was 84% (figure 1) and 86% for the total population. The 1y OS was 89% for c/pre-B-ALL and 75% for pro-B-ALL. OS was 100% at 1 yr for pts aged 55-65 yrs and 66% for those older than 65 yrs. 2 pts were transplanted in CR1. 2 pts relapsed, both pro-B-ALL, 1 pt developed a secondary malignancy (Colonic Ca) and 2 pts died in CR (1 HLH, 1 arterial disease). Disease Free Survival was 89% at 1 yr. Blinatumomab was well tolerated. No death occurred during Blina 1. The pt with HLH died after consolidation I but correlation to prior Blina is possible.
Overall tolerability and efficacy of the regimen was promising with a high cytologic and molecular response rate and low mortality for this age group. Dose-reduced chemo induction intended for leukemia debulking was effective but was also associated with two cases of ED. Age and subtype appeared to have an impact on the outcome with poorer results for older pts and those with pro-B-ALL. Confirmation of the results in a larger, potentially randomized trial and with longer follow-up is warranted.
Goekbuget: Incyte: Other: Research funding for Institution; Amgen: Consultancy, Other: Invited talks for company sponsored symposia (with honoraria); Research funding for institution; Astra Zeneca: Other: Invited talk for company sponsored symposia (with honor); Gilead/Kite: Consultancy; Novartis: Consultancy, Other: Research funding for Institution; Pfizer: Consultancy, Other: Research funding for institution; Jazz Pharmaceuticals: Other: Research funding for institution; Cellestia: Consultancy; Erytech: Consultancy; Morphosys: Consultancy; Servier: Consultancy, Other; Abbvie: Other. Topp: Macrogeniecs: Research Funding; Amgen: Consultancy, Research Funding; Regeneron: Consultancy, Research Funding; Gilead: Research Funding; Roche: Consultancy, Research Funding; Novartis: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy; Universitatklinikum Wurzburg: Current Employment. Schwartz: Novartis: Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau; Basilea: Other: Travel grants; MSD Sharp & Dohme: Membership on an entity's Board of Directors or advisory committees; BTG International Inc: Membership on an entity's Board of Directors or advisory committees; Morphosys: Research Funding; Gilead: Other: Travel grants, Speakers Bureau; Jazz Pharmaceuticals: Other: Travel grants, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau. Hertenstein: Novartis: Honoraria; Sanofi: Honoraria; Celgene: Honoraria; BMS: Honoraria. Vucinic: Gilead: Honoraria, Other: Travel Sponsoring; Abbvie: Honoraria, Other: Travel Sponsoring; MSD: Honoraria; Janssen: Honoraria, Other: Travel Sponsoring; Novartis: Honoraria. Brüggemann: Amgen: Other: Advisory Board, Travel support, Research Funding, Speakers Bureau; Incyte: Other: Advisory Board; Janssen: Speakers Bureau. Viardot: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; University Hospital of Ulm: Current Employment.
Blinatumomab in newly diagnosed ALL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal